Analysis of the Global Diabetes Drug Delivery Market
Analysis of the Global Diabetes Drug Delivery Market
Future Options for End Users
RELEASE DATE
17-Mar-2015
17-Mar-2015
REGION
Global
Global
Research Code: P85B-01-00-00-00
SKU: LS00146-GL-MR_16987
$4,950.00
Special Price $3,712.50 save 25 %
In stock
SKU
LS00146-GL-MR_16987
Description
The global diabetes drug delivery market is mainly divided into injectable drug delivery and oral drug delivery systems. The trend will continue in 2015, as four major products: Afrezza (inhalable drug delivery product of MannKind distributed by Sanofi), Tanzeum (injectable pen), Xigduo XR (oral), and Xultophy (injectable pen) enter the US and European diabetes markets. Depending on end-user acceptability of inhalable diabetes drugs, the global diabetes market can expect an increase in future investments. With the exception of one approved inhalable drug delivery system, there are no other inhalable products in the diabetes clinical pipeline at present.
RESEARCH: INFOGRAPHIC
This infographic presents a brief overview of the research, and highlights the key topics discussed in it.Click image to view it in full size
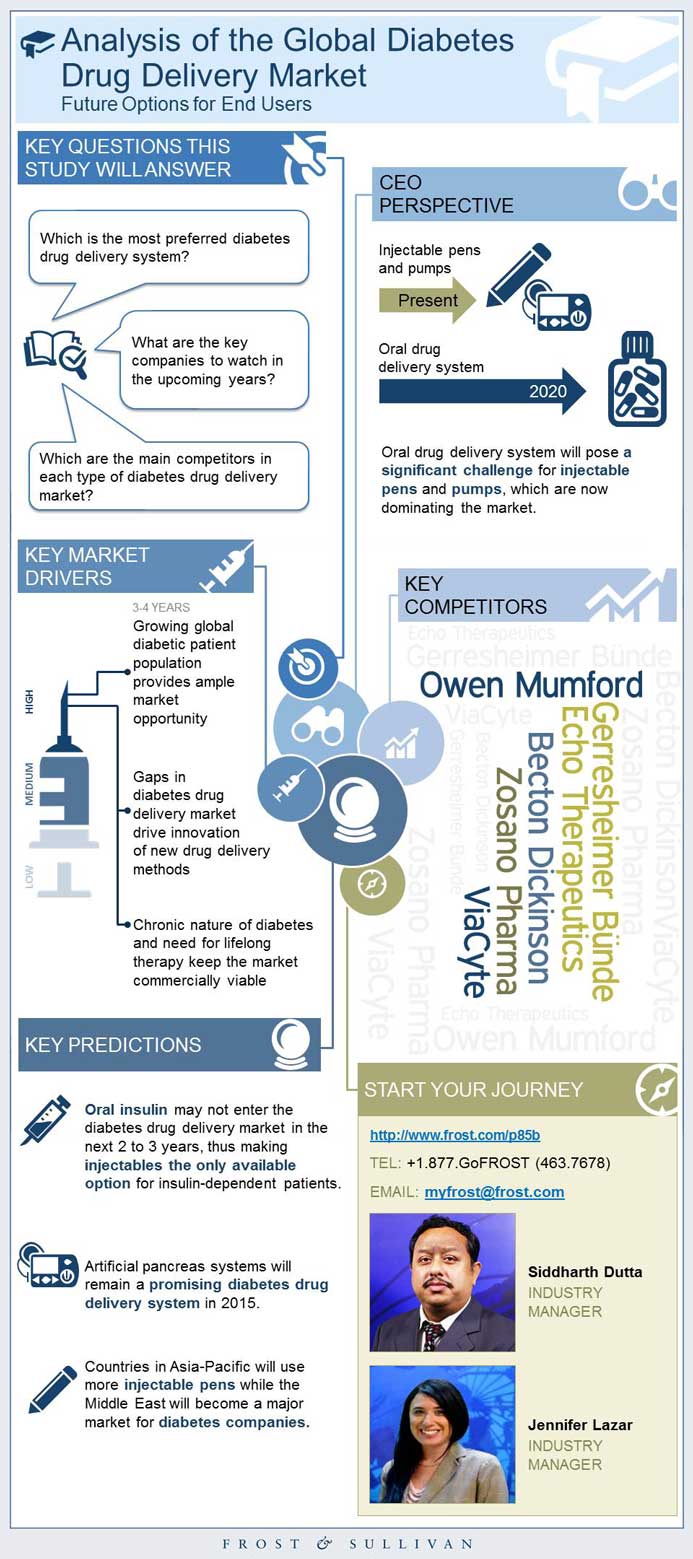
Table of Contents
Key Findings
Key Findings—Options in the Global Diabetes Drug Delivery Market
Unmet Needs in the Diabetes Drug Delivery Market
Pumps or Pens—Future Investment Opportunities for Type 1 Diabetes
Scope and Segmentation
Key Questions This Study Will Answer
Market Engineering Measurements
CEO’s Perspective
Key Companies to Watch
3 Key Predictions
Market Background
Market Segmentation
Market Segmentation (continued)
Global Diabetes Drug Delivery Market—Type of Delivery Usage by Region
New Market Opportunities
New Market Opportunities (continued)
Pipeline of Diabetes Drug Delivery Systems
Attractiveness in Diabetes Drug Delivery Market
Market Drivers
Market Restraints
Market Engineering Measurements
Forecast Assumptions
Revenue Forecast
Revenue Forecast by Type of Diabetes Drug Delivery
Revenue Forecast Discussion
Top Competitors
Market Engineering Measurements
Injectable Diabetes Drug Delivery Market—Revenue Forecast
Revenue Forecast Discussion
Injectable Diabetes Drug Delivery Market—Competitive Assessment
Promising Injectable—Once-a-week Diabetes Drug
Market Engineering Measurements
Oral Diabetes (Non-insulin) Drug Delivery Market—Revenue Forecast
Revenue Forecast Discussion
Oral Diabetes Drug Delivery Market—Competitive Assessment
Market Engineering Measurements
Inhalable Diabetes Drug Delivery Market—Revenue Forecast
Inhalable Diabetes Drug Delivery Market—Competitive Assessment
Inhalable Drug Delivery System and Injectable Drug Delivery System
The United States—New Technologies in Diabetes Drug Delivery
The United States—Trends in Diabetes Drug Delivery
Europe—New Technologies in Diabetes Drug Delivery
Europe—Trends in Diabetes Drug Delivery
APAC (India)—New Technologies in Diabetes Drug Delivery
APAC (India)—Trends in Diabetes Drug Delivery Market
Becton Dickinson and Company
Gerresheimer Bünde
Owen Mumford Ltd
Zosano Pharma Corporation
ViaCyte, Inc.
Echo Therapeutics
Conclusion
Frost & Sullivan Awards
Legal Disclaimer
Market Engineering Methodology
Drivers Explained
Drivers Explained (continued)
Restraints Explained
Learn More—Next Steps
Popular Topics
The global diabetes drug delivery market is mainly divided into injectable drug delivery and oral drug delivery systems. The trend will continue in 2015, as four major products: Afrezza (inhalable drug delivery product of MannKind distributed by Sanofi), Tanzeum (injectable pen), Xigduo XR (oral), and Xultophy (injectable pen) enter the US and European diabetes markets. Depending on end-user acceptability of inhalable diabetes drugs, the global diabetes market can expect an increase in future investments. With the exception of one approved inhalable drug delivery system, there are no other inhalable products in the diabetes clinical pipeline at present.
| No Index | No |
|---|---|
| Podcast | No |
| Author | Siddharth Dutta |
| WIP Number | P85B-01-00-00-00 |
| Is Prebook | No |
 USD
USD GBP
GBP CNY
CNY EUR
EUR INR
INR JPY
JPY MYR
MYR ZAR
ZAR KRW
KRW THB
THB