Analysis of the US Retinal Therapeutics Market
Analysis of the US Retinal Therapeutics Market
Improvements in Administration and Efficacy Drive Growth
RELEASE DATE
11-Dec-2013
11-Dec-2013
REGION
Global
Global
Research Code: NC77-01-00-00-00
SKU: LS00115-GL-MR_16956
$4,950.00
Special Price $3,712.50 save 25 %
In stock
SKU
LS00115-GL-MR_16956
Description
Since the introduction of Lucentis in 2006, vascular endothelial growth factor (VEGF) antagonists have become the clear choice in treatment for age related macular degeneration, diabetic macular edema, and retinal vein occlusion due to their high efficacy and safety. This research service evaluates the US market for the pharmaceutical management of AMD, DME, and RVO from 2009 to 2017, with 2012 as the base year. Segmentation by disorder is provided. Included are products on the market, products in development, and patient and revenue forecasts. Market challenges, drivers, and restraints are identified and assessed.
Table of Contents
Key Findings
Key Findings (continued)
Scope and Segmentation
Key Questions This Study Will Answer
Market Engineering Measurements
Market Engineering Measurements (continued)
CEO’s Perspective
Key Companies to Watch
Executive Summary—3 Big Predictions
Market Segmentation
Compounding Pharmacies
New Market Opportunities
New Market Opportunities (continued)
Market Drivers
Market Restraints
Market Engineering Measurements
Market Engineering Measurements (continued)
Market Engineering Measurements (continued)
Forecast Assumptions and Definitions
Revenue Forecast
Revenue Forecast Discussion
Pricing Trends and Forecast
Pricing Trends and Forecast Discussion
Competitive Landscape
Marketed Product Analysis
Pipeline Analysis
Pipeline Analysis (continued)
Pipeline Analysis (continued)
Market Share
Market Share Evolution
Market Share Analysis
Top Competitors
Timeline of Key Events
Patent Expirations
Comparative Efficacy Analysis
Comparative Efficacy Analysis Discussion
AMD Segment—Market Engineering Measurements
AMD Segment—Revenue Forecast
AMD Segment—Revenue Forecast Discussion
AMD Segment—Prevalence and Treated Patients Forecast
AMD Segment—Prevalence and Treated Patients Forecast Discussion
AMD Segment—Penetration Analysis
AMD Segment—Penetration Analysis (continued)
DME Segment Market Engineering Measurements
DME Segment—Revenue Forecast
DME Segment—Revenue Forecast Discussion
DME Segment—Prevalence and Treated Patients Forecast
DME Segment—Prevalence and Treated Patients Forecast Discussion
DME Segment—Penetration Analysis
DME Segment—Penetration Analysis (continued)
RVO Segment—Market Engineering Measurements
RVO Segment—Revenue Forecast
RVO Segment—Revenue Forecast Discussion
RVO Segment—Prevalence and Treated Patients Forecast
RVO Segment—Prevalence and Treated Patients Discussion
RVO Segment—Penetration Analysis
RVO Segment Penetration Analysis (continued)
Companies to Watch
Regeneron
Ophthotec
Allergan
The Last Word—Three Big Predictions
The Last Word—Discussion
The Last Word—Discussion (continued)
The Last Word—Discussion (continued)
Legal Disclaimer
Drivers Explained
Drivers Explained (continued)
Drivers Explained (continued)
Drivers Explained (continued)
Drivers Explained (continued)
Restraints Explained
Restraints Explained (continued)
Restraints Explained (continued)
Additional Sources of Information on Ophthalmic Disorders
Market Engineering Methodology
Learn More—Next Steps
Popular Topics
The global diabetes drug delivery market is mainly divided into injectable drug delivery and oral drug delivery systems. The trend will continue in 2015, as four major products: Afrezza (inhalable drug delivery product of MannKind distributed by Sanofi), Tanzeum (injectable pen), Xigduo XR (oral), and Xultophy (injectable pen) enter the US and European diabetes markets. Depending on end-user acceptability of inhalable diabetes drugs, the global diabetes market can expect an increase in future investments. With the exception of one approved inhalable drug delivery system, there are no other inhalable products in the diabetes clinical pipeline at present. --BEGIN PROMO-- 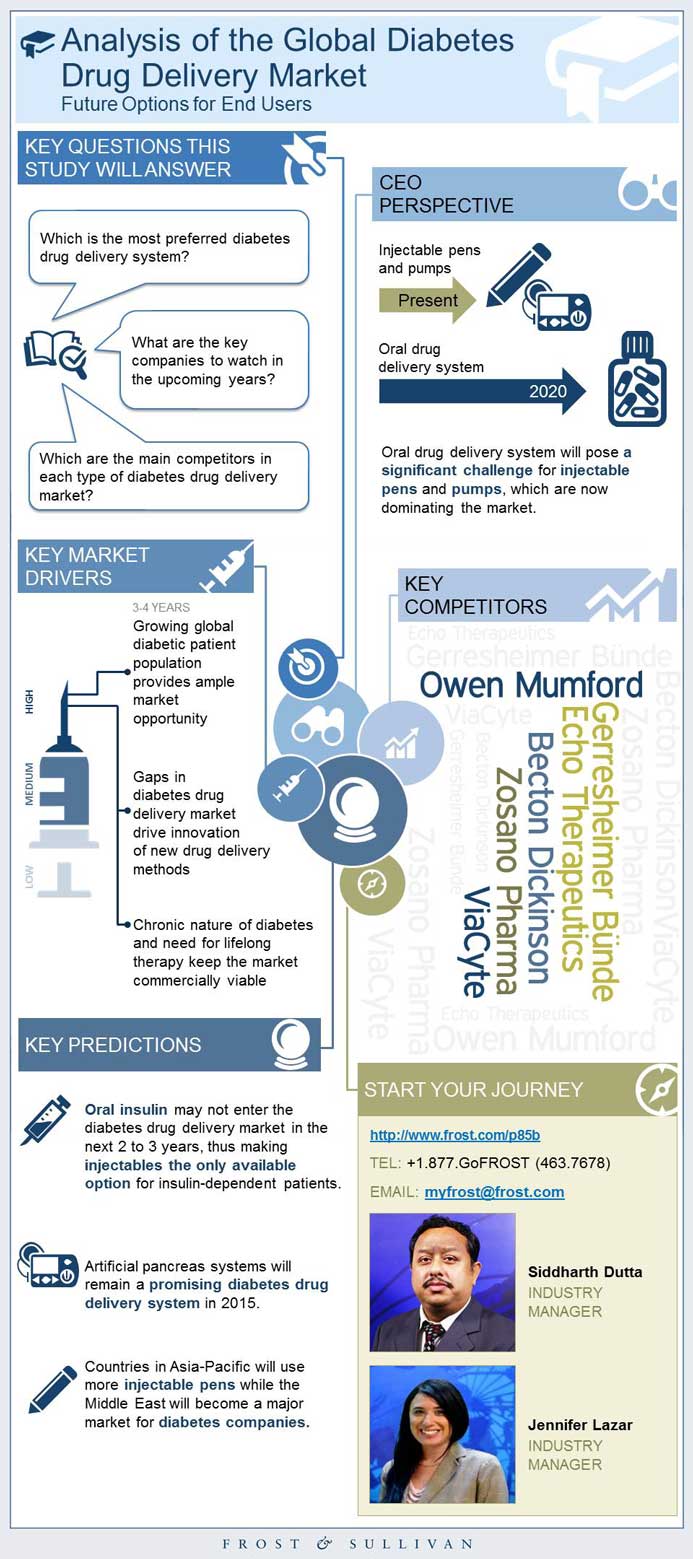

| No Index | No |
|---|---|
| Podcast | No |
| Author | Deborah Toscano |
| WIP Number | NC77-01-00-00-00 |
| Is Prebook | No |
 USD
USD GBP
GBP CNY
CNY EUR
EUR INR
INR JPY
JPY MYR
MYR ZAR
ZAR KRW
KRW THB
THB