Product and Pipeline Assessment of the Global Orphan Drugs Market
Product and Pipeline Assessment of the Global Orphan Drugs Market
Attractive Market Lures Hundreds of Innovators, Large and Small
RELEASE DATE
27-Feb-2014
27-Feb-2014
REGION
Asia Pacific
Asia Pacific
Deliverable Type
Market Research
Market Research
Research Code: ND7D-01-00-00-00
SKU: LS00120-AP-MR_16961
$3,950.00
In stock
SKU
LS00120-AP-MR_16961
Breakthroughs in drug discovery have led to rich and competitive pipelines for rare diseases such as rare cancers as well as neurologic disorders and musculoskeletal disorders, among many other therapeutic areas. This research service provides a comprehensive global overview and analysis of global orphan drugs development, with an emphasis on the US market. Included are market background and global overview and analysis of drug development and regulatory activity by disease area and geography, detailed product and pipeline analysis of orphan drugs by therapeutic area and disease, and company analysis, including activity analysis and key companies to watch.
Executive Summary
Executive Summary (continued)
Methodology and Scope
Market Background
Market Background (continued)
Market Background (continued)
NIH Orphan Diseases by Type
NIH Orphan Diseases by Type (continued)
Global Overview and Analysis
Global Overview and Analysis (continued)
Orphan Diseases with FDA-approved Therapies
Orphan Diseases with FDA-approved Therapies (continued)
Orphan Diseases with FDA-approved Therapies (continued)
Orphan Diseases with FDA-approved Therapies (continued)
Orphan Diseases with FDA-approved Therapies (continued)
Orphan Diseases with FDA-approved Therapies (continued)
Orphan Diseases with FDA-approved Therapies (continued)
FDA Approvals and Designations by Therapeutic Area
FDA Orphan Designations by Therapeutic Area
EMA Orphan Designations by Therapeutic Area
Count of US FDA and European EMA Orphan Drug Designations by Year
Count of US FDA and European EMA Orphan Drug Approvals by Year
Count of US FDA Orphan Drug Designations and Approvals by Year
Count of European EMA Orphan Drug Designations and Approvals by Year
Top Rare Cancer Designations
Top Rare Cancer Designations (continued)
Annual Count of FDA Rare Cancer Designations
Annual Count of FDA Pediatric Orphan Designations
Top Indications by Activity and Disease Overview
SCD—Orphan Drugs on the Market
SCD—Orphan Drugs in Development
SCD—Orphan Drugs in Development (continued)
MDS—Orphan Drugs on the Market
MDS—Orphan Drugs in Development
TTP—Orphan Drugs in Development
Top Indications by Activity and Disease Overview
PAH—Orphan Drugs on the Market
PAH—Orphan Drugs in Development
PAH—Orphan Drugs in Development (continued)
Calciphylaxis—Orphan Drugs in Development
Top Indications by Activity and Disease Overview
CF—Orphan Drugs on the Market
CF—Orphan Drugs in Development
CF—Orphan Drugs in Development (continued)
CF—Orphan Drugs in Development (continued)
CF—Orphan Drugs in Development (continued)
IPF—Orphan Drugs on the Market
IPF—Orphan Drugs in Development
IPF—Orphan Drugs in Development (continued)
IPF—Orphan Drugs in Development (continued)
Top Indications by Activity and Disease Overview
Fragile X Syndrome—Orphan Drugs in Development
HIBM—Orphan Drugs in Development
Top Indications by Activity and Disease Overview
SSc—Orphan Drugs in Development
EB—Orphan Drugs in Development
Top Indications by Activity and Disease Overview
Ped CD—Orphan Drugs on the Market
Ped CD—Orphan Drugs in Development
Ped CD—Orphan Drugs in Development (continued)
Ped UC—Orphan Drugs on the Market
Ped UC—Orphan Drugs in Development
Ped UC—Orphan Drugs in Development (continued)
Pouchitis—Orphan Drugs in Development
Top Indications by Activity and Disease Overview
T1D with Residual Beta-cell Function—Orphan Drugs in Development
T1D with Residual Beta-cell Function—Orphan Drugs in Development (continued)
Top Indications by Activity and Disease Overview
ARS—Orphan Drugs in Development
ARS—Orphan Drugs in Development (continued)
Top Indications by Activity and Disease Overview
RP—Orphan Drugs in Development
RP—Orphan Drugs in Development (continued)
Stargardt Disease—Orphan Drugs in Development
Uveitis—Orphan Drugs on the Market
Uveitis—Orphan Drugs in Development
Top Indications by Activity and Disease Overview
Behcet's Disease—Orphan Drugs in Development
Pipeline Analyses for Select Rare Diseases—Infectious/Parasitic Diseases
Top Indications by Activity and Disease Overview
CMV Infection—Orphan Drugs on the Market
CMV Infection—Orphan Drugs in Development
Malaria—Orphan Drugs on the Market
Malaria—Orphan Drugs in Development
Leishmaniasis—Orphan Drugs on the Market
Leishmaniasis—Orphan Drugs in Development
Top Indications by Activity and Disease Overview
Top Indications by Activity and Disease Overview (continued)
MPS—Orphan Drugs on the Market
MPS—Orphan Drugs in Development
MPS—Orphan Drugs in Development (continued)
Acromegaly—Orphan Drugs on the Market
Acromegaly—Orphan Drugs in Development
Urea Cycle Disorders—Orphan Drugs on the Market
Urea Cycle Disorders—Orphan Drugs in Development
NPC Disease—Orphan Drugs on the Market
NPC Disease—Orphan Drugs in Development
Pompe Disease—Orphan Drugs on the Market
Pompe Disease—Orphan Drugs in Development
Top Indications by Activity and Disease Overview
DMD—Orphan Drugs in Development
DMD—Orphan Drugs in Development (continued)
DMD—Orphan Drugs in Development (continued)
DMD—Orphan Drugs in Development (continued)
SMA—Orphan Drugs in Development
Top Indications by Activity and Disease Overview
ALS—Orphan Drugs in Development
ALS—Orphan Drugs in Development (continued)
ALS—Orphan Drugs in Development (continued)
ALS—Orphan Drugs in Development (continued)
HD—Orphan Drugs on the Market
HD—Orphan Drugs in Development
FA—Orphan Drugs in Development
Top Indications by Activity and Disease Overview
Pancreatic Cancer—Orphan Drugs on the Market
Pancreatic Cancer—Orphan Drugs in Development
Pancreatic Cancer—Orphan Drugs in Development (continued)
Pancreatic Cancer—Orphan Drugs in Development (continued)
Pancreatic Cancer—Orphan Drugs in Development (continued)
Pancreatic Cancer—Orphan Drugs in Development (continued)
Pancreatic Cancer—Orphan Drugs in Development (continued)
Glioma—Orphan Drugs on the Market
Glioma—Orphan Drugs in Development
Glioma—Orphan Drugs in Development (continued)
Glioma—Orphan Drugs in Development (continued)
Glioma—Orphan Drugs in Development (continued)
Glioma—Orphan Drugs in Development (continued)
AML—Orphan Drugs on the Market
AML—Orphan Drugs in Development
AML—Orphan Drugs in Development (continued)
AML—Orphan Drugs in Development (continued)
AML—Orphan Drugs in Development (continued)
AML—Orphan Drugs in Development (continued)
Top 20 Companies by Activity
Top Companies by Activity per Therapeutic Area
Top Earning Orphan Drugs
Select Recent Launches
Companies to Watch—Alexion Pharmaceuticals
Companies to Watch—BioMarin Pharmaceutical Inc.
Companies to Watch—Celgene Corporation
Companies to Watch—PTC Therapeutics
Companies to Watch—Spotlight on Innovation
Strategic Partnership Assessment
Strategic Partnership Assessment (continued)
Strategic Partnership Assessment (continued)
Conclusions and Recommendations
Legal Disclaimer
List of Rare Cancers in “Others”
List of Rare Cancers in “Others” (continued)
List of Rare Cancers in “Others” (continued)
The Frost & Sullivan Story
Value Proposition: Future of Your Company & Career
Global Perspective
Industry Convergence
360º Research Perspective
Implementation Excellence
Our Blue Ocean Strategy
Purchase includes:
- Report download
- Growth Dialog™ with our experts
Growth Dialog™
A tailored session with you where we identify the:- Strategic Imperatives
- Growth Opportunities
- Best Practices
- Companies to Action
Impacting your company's future growth potential.
The global market for vaccines is estimated to be only 2-3% of the total pharmaceuticals market in 2014; however, this sector has been continuously experiencing a stellar growth rate of 10-15% per year versus the 5-7% seen in the overall market. Currently, the most commonly available vaccines are administered by injection, which makes mass immunization more costly and less safe, particularly in resource-poor developing countries. More than 120 new products are currently in the development pipeline, of which sixty hold significant importance to developing nations. The type of delivery system will predict which type of vaccine delivery will remain the most attractive (in terms of revenue) for stakeholders in the next 5 years. --BEGIN PROMO-- 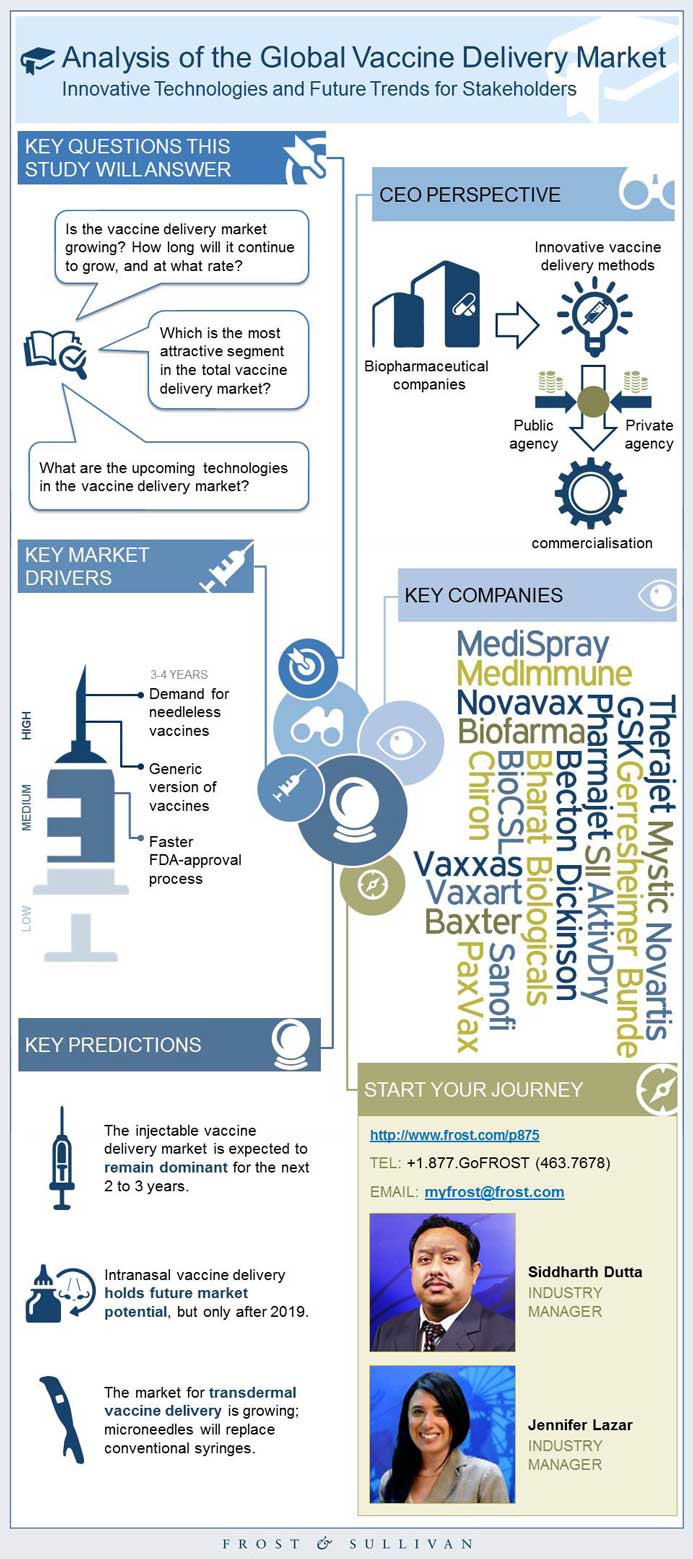

| Deliverable Type | Market Research |
|---|---|
| No Index | Yes |
| Podcast | No |
| Author | Deborah Toscano |
| WIP Number | ND7D-01-00-00-00 |
| Is Prebook | No |