Global Cell and Gene Therapy (CGT) Growth Opportunities
Global Cell and Gene Therapy (CGT) Growth Opportunities
The Shift to Prevalent Diseases and Allogeneic Cell Therapies Creates New Growth Opportunities in the CGT Value Chain
16-Aug-2022
North America
Market Research
Description
This Frost & Sullivan study examines the global cell and gene therapy (CGT) market. It highlights revenue forecasts and key growth opportunities for CGT market participants based on the evolution of their business models and strategic approaches as well as investments and regulations. The study segments the market by product (cell therapy, gene-modified cell therapy, and gene therapy), and it also focuses on subsegments (allogeneic versus autologous; ex vivo versus in vivo). Market forecasts run from 2022 through 2027, and they capture key market developments, such as capacity expansion, mergers and acquisitions, and extension of services offerings set to impact overall CGT market growth. As CGT moves from ultra-rare indications to more common indications, the rapid expansion of manufacturing capabilities to support the commercial requirements of these diseases will become a necessity. The migration from paper-based data to the digital format will connect individual patient supply chain data with individual patient clinical data and incorporate it into the overall analysis. Time and speed will become crucial factors to stay ahead of the competition and reap the resulting benefits. The success of CGT in prevalent diseases, such as diabetes and stroke, will be the game changer during the forecast period and lead to new market access models.
The research service also covers the following topics:
• Market and Pipeline Snapshot
• Key Trends and Value Chain Analysis
• CGT Investment and M&A Snapshot
• CGT Reimbursement Snapshot
• CGT Regulatory Snapshot
• Drivers and Restraints
• Recent Acquisitions and Capacity Expansion by Major Bio-CDMOs
Author: Surbhi Gupta
RESEARCH: INFOGRAPHIC
This infographic presents a brief overview of the research, and highlights the key topics discussed in it.Click image to view it in full size
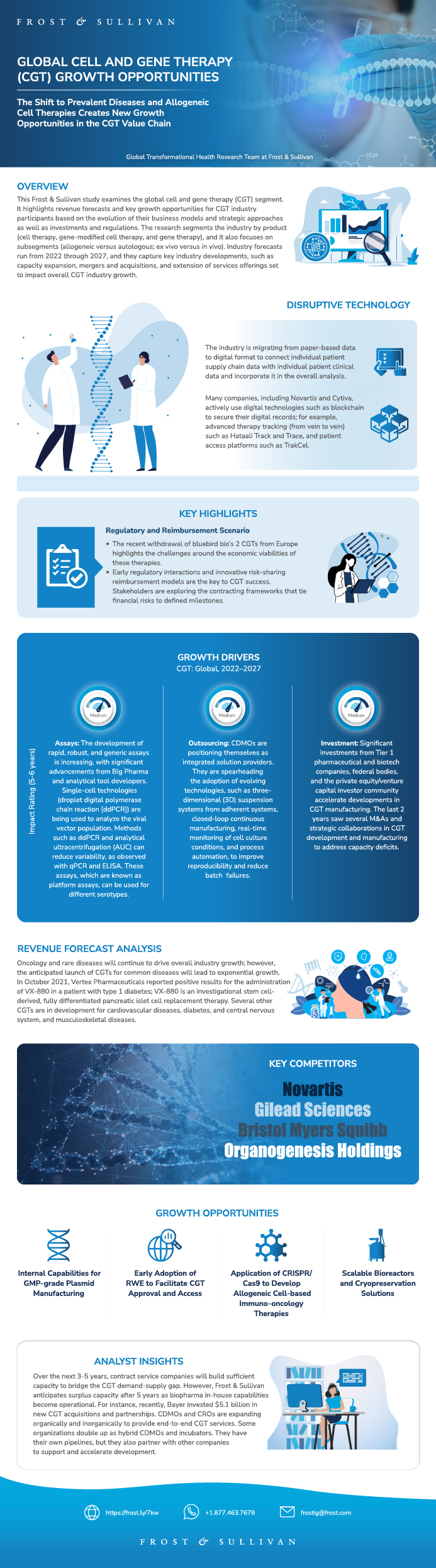
Table of Contents
Why Is It Increasingly Difficult to Grow?
The Strategic Imperative 8™
The Impact of the Top 3 Strategic Imperatives on the Global CGT Industry
Growth Opportunities Fuel the Growth Pipeline Engine™
Key Highlights
Scope of Analysis
Segmentation
Definitions
Growth Metrics
Growth Drivers
Growth Restraints
Forecast Assumptions
Revenue Forecast
Revenue Forecast by Product
Revenue Forecast Analysis
Revenue Forecast by Region
Revenue Forecast Analysis by Region
Competitive Environment
Revenue Share
Revenue Share Analysis
Key Approved CGTs Until Q1 2022
Key Approved CGTs Until Q1 2022 (continued)
Upcoming Regulatory Approvals in the US and the EU
Pipeline Snapshot
Gene Therapy and Genetically Modified Cell Therapies: Pipeline Snapshot
Cell Therapies: Pipeline Snapshot
CGT for Cancer: A Snapshot
CGT Value Chain
CGT Stakeholder Ecosystem
Traditional Drugs versus CGT Business Models
Evolving CGT Business Model: Orchestrated Collaborations between Stakeholders across the Value Chain
Key CGT Stakeholders’ Portfolio Strategies
CGT Value Chain: Evolving Role of Stakeholders in Digitalization
Winning Strategies for CGT Companies
Key Trends Driving Investment, Expansion, Partnerships, and M&As
Key Trends Driving Investment, Expansion, Partnerships, and M&As (continued)
Trends in CGT Investment, 2021
Notable Start-up Financing, 2021
Notable M&As, 2021–Q1 2022
Current Reimbursement Approach for CGTs: US and EU5
Current Reimbursement Approach for CGTs: APAC
Examples of Innovative CGT Reimbursement Models
Current Formal Regulatory Meetings with US and EU Regulatory Agencies
Approval Exemptions for CGT
Early Access Programs for CGT: Facilitated Approval
Early Access Programs for CGT: Priority Review
Early Access Programs for CGT: Priority Designation
Early Access Programs for CGT: Orphan Drug Designation
Regulatory Risk Assessment in Early Development
China’s Regulatory Environment has Drastically Evolved and Became Supportive for CGT Development, Leading to the Expansion of the Domestic CGT Industry
Growth Metrics, Cell Therapy
Revenue Forecast: Cell Therapy
Revenue Forecast by Source
Revenue Forecast Analysis
Growth Metrics, Gene Therapy
Revenue Forecast: Gene Therapy
Revenue Forecast by Technology
Revenue Forecast Analysis
Growth Metrics, Gene-modified Cell Therapy
Revenue Forecast: Gene-modified Cell Therapy
Revenue Forecast by Source
Revenue Forecast Analysis
Growth Opportunity 1: Internal Capabilities for GMP-grade Plasmid Manufacturing
Growth Opportunity 1: Internal Capabilities for GMP-grade Plasmid Manufacturing (continued)
Growth Opportunity 2: Early Adoption of RWE to Facilitate CGT Approval and Access
Growth Opportunity 2: Early Adoption of RWE to Facilitate CGT Approval and Access (continued)
Growth Opportunity 3: Application of CRISPR/Cas9 to Develop Allogeneic Cell-based Immuno-oncology Therapies
Growth Opportunity 3: Application of CRISPR/Cas9 to Develop Allogeneic Cell-based Immuno-oncology Therapies (continued)
Growth Opportunity 4: Scalable Bioreactors and Cryopreservation Solutions
Growth Opportunity 4: Scalable Bioreactors and Cryopreservation Solutions (continued)
Your Next Steps
Why Frost, Why Now?
List of Exhibits
List of Exhibits (continued)
Legal Disclaimer
| Deliverable Type | Market Research |
|---|---|
| Author | Surbhi Gupta |
| Industries | Healthcare |
| No Index | No |
| Is Prebook | No |
| Keyword 1 | CGT Market |
| Keyword 2 | Cell and Gene Therapy |
| Podcast | No |
| WIP Number | PD67-01-00-00-00 |
 USD
USD GBP
GBP CNY
CNY EUR
EUR INR
INR JPY
JPY MYR
MYR ZAR
ZAR KRW
KRW THB
THB