Global Contract Research Organization (CRO) Growth Opportunities
Global Contract Research Organization (CRO) Growth Opportunities
Strong Competitive Intensity is Mandating a Greater Industry Convergence Resulting in a Strong Market Growth
11-Nov-2022
North America
Market Research
Description
The COVID-19 pandemic led to a sudden surge in R&D activities across the world. Both small and large pharmaceutical and biotech companies were engaged in tremendous efforts to either develop new molecules or repurpose existing therapies as potential drugs for COVID-19 treatment.
The trend is likely to continue with small-to-medium-segment and emerging biopharma participants introducing novel therapies across various indications, primarily oncology, infectious diseases, and neurology. Per Pharma Intelligence's annual R&D review, global non-clinical and clinical pipelines have grown by leaps and bounds--more than 10,000 molecules were in preclinical development and about 6,000 molecules were in clinical development as of January 2022.
Industry dynamics saw a huge shift with much of the clinical research being conducted in emerging economies and third-world countries, such as India, Indonesia, China, LATAM, the Middle East, and South Africa. As a result, several local CROs expanded their market reach by partnering with larger, global CROs and providing local clinical research support. The industry also moved to adopt large-scale decentralized clinical trials, with a year-over-year growth of 50% and 28% between 2020-2021 and 2021-2022, respectively. Such changes expanded the participation of eClinical solution vendors and encouraged collaborations with CROs and pharmaceutical companies, providing cutting-edge solutions for data capture, pharmacovigilance, trial management, and other applications.
Drug discovery and preclinical service outsourcing (traditionally conducted in house because of IP infringement issues) has increased, owing to
the rise in specialized drug discovery and preclinical CROs and the CRDMO model gaining traction. CROs work as risk-sharing partners and offer one-stop-shop services to pharmaceutical companies, thereby cutting down costs and shortening the timeline of development.
Key Issues Addressed:
- What are the general industry trends pertaining to the global CRO industry?
- What are the key drivers triggering large-scale outsourcing of both early- and late-stage nonclinical and clinical development?
- What are the key emerging business models providing a competitive advantage to pharmaceutical sponsors and how is it supporting industry convergence?
- Who are the leading participants driving market growth?
- What is the opportunity for small- to medium-segment CRO participants in a sea of competitors?
- How is the application of technology supporting the growth of the CRO market and, in general, the drug development outsourcing services?
Author: Aarti Siddhesh Chitale
RESEARCH: INFOGRAPHIC
This infographic presents a brief overview of the research, and highlights the key topics discussed in it.Click image to view it in full size
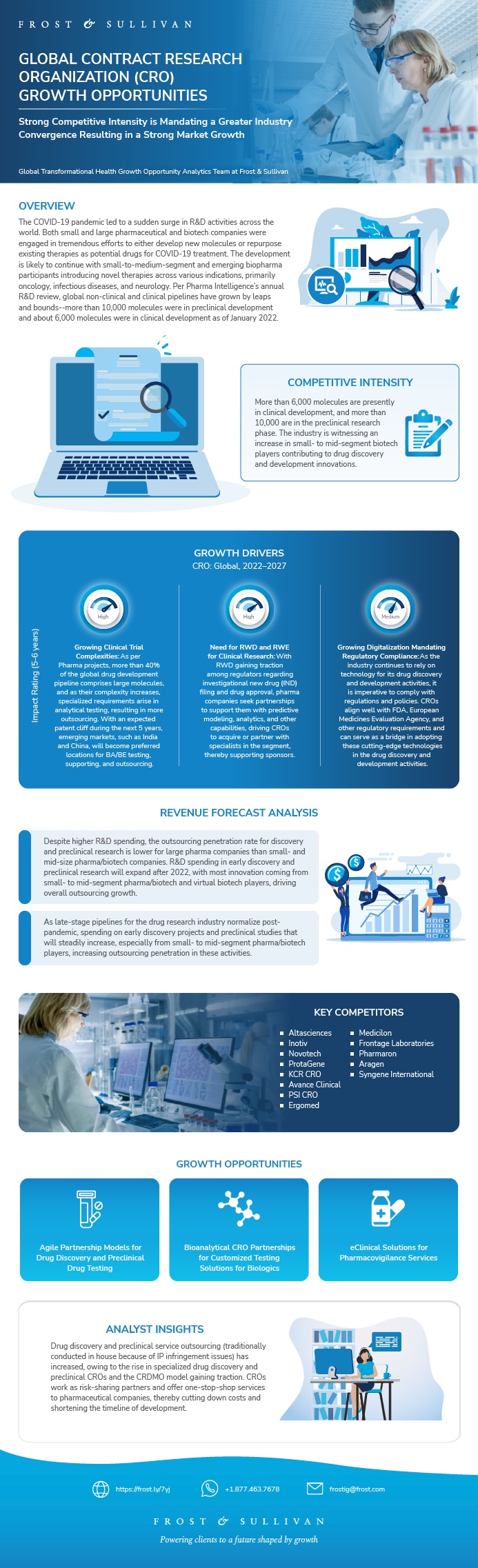
Table of Contents
Why is it Increasingly Difficult to Grow?
The Strategic Imperative 8™
The Impact of the Top 3 Strategic Imperatives on the Contract Research Organization (CRO) Industry
Growth Opportunities Fuel the Growth Pipeline Engine™
Scope of Analysis
Segmentation
Peripheral Segments
Market Trend Analysis
Market Trend Analysis (continued)
Vendor Ecosystem
Growth Drivers
Growth Restraints
Services and Stakeholders
CRDMO—The Future of Outsourcing
CRDMO—The Future of Outsourcing (continued)
CRDMO Transactions to Support Drug Discovery and Development
CRDMO Transactions to Support Drug Discovery and Development (continued)
Digitization of the Drug Development Value Chain
Digitization of Clinical Research
DCTs—Future Impact on the CRO Industry
Investment Trends—Collaborative Approach for Drug Development
Early- to Late-stage CRO Service Collaboration—Nonclinical
Early- to Late-stage CRO Service Collaboration—Nonclinical (continued)
Early- to Late-stage CRO Service Collaboration—Nonclinical (continued)
Early- to Late-stage CRO Service Collaboration—Clinical
Early- to Late-stage CRO Service Collaboration—Clinical (continued)
Early- to Late-stage CRO Service Collaboration—Clinical (continued)
Technology Partnerships Supporting Industry Convergence
Technology Partnerships Supporting Industry Convergence (continued)
Technology Partnerships Supporting Industry Convergence (continued)
Expanding CRO Expertise through Industry Acquisitions
Expanding CRO Expertise through Industry Acquisitions (continued)
Expanding CRO Expertise through Industry Acquisitions (continued)
Expanding CRO Expertise through Industry Acquisitions (continued)
Expanding CRO Expertise through Industry Acquisitions (continued)
Expanding CRO Expertise through Industry Acquisitions (continued)
Growth Metrics
Forecast Assumptions
Forecast Assumptions (continued)
Revenue Forecast
Revenue Forecast—Clinical vs. Nonclinical
Revenue Forecast Analysis
Revenue Forecast Analysis (continued)
Growth Metrics
Revenue Forecast Assumptions and Methodology
Key Services—Drug Discovery and Preclinical Research,
Nuances of Drug Discovery and Preclinical Development
Pharmaceutical R&D Expenditure
Revenue Forecast
Revenue Forecast by Nonclinical Phases of Development
Revenue Forecast Analysis
Revenue Forecast Analysis (continued)
Revenue Forecast Analysis (continued)
Growth Metrics
Revenue Forecast Assumptions
Revenue Forecast Methodology
Revenue Forecast
Revenue Forecast by Clinical Phases of Development
Percent Revenue Forecast by Clinical Phases of Development
Revenue Forecast Analysis
Revenue Forecast Analysis (continued)
Clinical Trial Split by Therapy Area
Revenue Share Analysis By Region
North America—Key Trends
North America—Revenue Forecast by Clinical Development Phase
North America—Revenue Forecast Analysis
Europe—Key Trends
Europe—Revenue Forecast by Clinical Development Phase
Europe—Revenue Share Analysis
APAC—Key Trends
APAC—Revenue Forecast by Clinical Development Phases
APAC—Revenue Share Analysis
RoW—Key Trends
RoW—Revenue Forecast by Clinical Development Phase
RoW—Revenue Share Analysis
Competitive Environment
Key Competitors
Revenue Share
Revenue Share Analysis
Revenue Share Analysis (continued)
Cost Split by Service Type
Peripheral Services—Analytical Testing Services
Peripheral Services—Clinical Outcomes and Post-market Surveillance
Peripheral Services—Data Capture and Management
Growth Opportunity 1: Agile Partnership Models for Drug Discovery and Preclinical Drug Testing
Growth Opportunity 1: Agile Partnership Models for Drug Discovery and Preclinical Drug Testing (continued)
Growth Opportunity 2: Bioanalytical CRO Partnerships for Customized Testing Solutions for Biologics
Growth Opportunity 2: Bioanalytical CRO Partnerships for Customized Testing Solutions for Biologics (continued)
Growth Opportunity 3: eClinical Solutions for Pharmacovigilance Services
Growth Opportunity 3: eClinical Solutions for Pharmacovigilance Services (continued)
Growth Opportunity 4: AI-enabled Oncology Trial Design, Recruitment, and Execution
Growth Opportunity 4: AI-enabled Oncology Trial Design, Recruitment, and Execution (continued)
Your Next Steps
Why Frost, Why Now?
List of Exhibits
List of Exhibits (continued)
Legal Disclaimer
Popular Topics
Key Issues Addressed:
- What are the general industry trends pertaining to the global CRO industry
- What are the key drivers triggering large-scale outsourcing of both early- and late-stage nonclinical and clinical development
- What are the key emerging business models providing a competitive advantage to pharmaceutical sponsors and how is it supporting industry convergence
- Who are the leading participants driving market growth
- What is the opportunity for small- to medium-segment CRO participants in a sea of competitors
- How is the application of technology supporting the growth of the CRO market and, in general, the drug development outsourcing services
Author: Aarti Siddhesh Chitale
| Deliverable Type | Market Research |
|---|---|
| Author | Aarti Siddhesh Chitale |
| Industries | Healthcare |
| No Index | No |
| Is Prebook | No |
| Podcast | No |
| WIP Number | PDC6-01-00-00-00 |
 THB
THB GBP
GBP CNY
CNY EUR
EUR INR
INR JPY
JPY MYR
MYR ZAR
ZAR KRW
KRW USD
USD